Pfizer Recorded 1,223 Possible Vaccine Deaths During First 90 Days of COVID Vaccine Rollout
A Freedom of Information Act (FOIA) request made by the Public Health and Medical Professionals for Transparency group has revealed that Pfizer was aware of 1,223 possible vaccine related deaths within the first 90 days of their COVID vaccine rollout. It's quite clear that Pfizer did not find any causal relationship to the vaccine with regards to these deaths. This means that it was not determined that the vaccine did or did not play a factor in these deaths. We explain why this is the case, below.
The Public Health and Medical Professionals for Transparency group is comprised of approximately 30 professors and scientists who are passionate about medical transparency - something we shouldn't have to fight for.
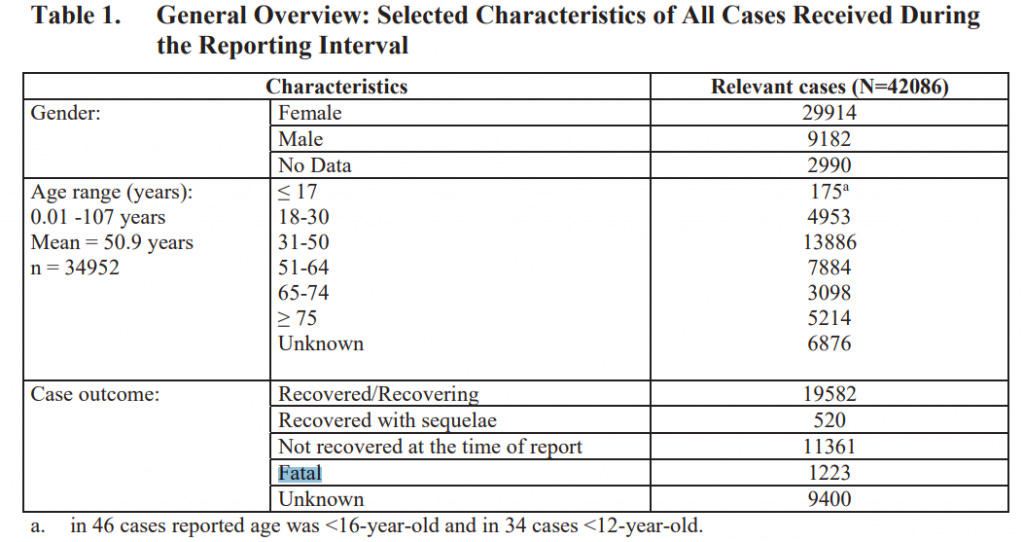
Table 1. in Pfizer's documents accounts 42,086 reactions to their COVID-19 vaccine over a 90-day period from December 1st. 2020 – February 28th, 2021. 25,379 reactions were 'medically confirmed' while 16,707 were 'not-medically confirmed.' Pfizer also redacted the total number of vaccine doses that had been administered up to this point.
According to Pfizer, an event that's not medically confirmed comes as a result of reports that come in without medical records and other necessary documents to make a confirmation.
Pfizer also notes that,
"In some reports, clinical information (such as medical history, validation of diagnosis, time from drug use to onset of illness, dose, and use of concomitant drugs) is missing or incomplete, and follow-up information may not be available."
"An accumulation of adverse event reports (AERs) does not necessarily indicate that a particular AE was caused by the drug; rather, the event may be due to an underlying disease or some other factor(s) such as past medical history or concomitant medication."
Pfizer Document.
The documents have also revealed that Pfizer was well aware of tens of thousands of other adverse and serious adverse reactions within months of distribution. This includes 1,403 cases of cardiovascular issues and many cases of vaccine induced heart complications.
The FOIA request to obtain these documents was made to the Food and Drug Administration (FDA), who at first did not release them. A court order forced them to comply and begin releasing documents. A joint court order explains that the FDA has proposed to produce 500 pages per month. Based on its calculated number of pages, this would mean that it would complete its production in nearly 55 years, in 2076. This is how long it would take for the public to have clarity on why the FDA approved these vaccines.
"Until the entire body of documents provided by Pfizer to the FDA are made available, an appropriate analysis by the independent scientists that are members of Plaintiff is not possible. Would the FDA agree to review and license this product without all the documents? Of course not. These independent, world-renowned scientists should be provided the same forthwith."
This is not a surprise, a systematic review in PLOS journal analysed 28 studies and found that adverse events were less likely to appear in published journal articles than unpublished studies (e.g. industry-held data).
Other concerning data from the Pfizer trials was outlined by whistleblower testimony of Brooke Jackson. An article in the British Medical Journal brought light to her claims that some Pfizer COVID vaccine trial data was falsified. The company she worked for, Ventavia, one of the companies contracted to perform vaccine trials for Pfizer, even tried to discredit her claims as false by saying she did not work on the Pfizer trials. But later released documents showed Ventavia was lying.
More documents pertaining to the Pfizer data are set to be released in the coming weeks. Public Health and Medical Professionals for Transparency filed another motion that would force the FDA to expedite the release of requested documents as well.
Despite the findings outlined in this initial batch of documents, the FDA still thought it was necessary and appropriate to authorize emergency use for these products. In doing so, those who have been, and were injured, by COVID vaccines are not eligible for compensation via the Vaccine Injury Compensation Program.
To date, the program has shielded pharmaceutical companies from liability while payouts to victims come from taxpayer dollars. To date the program has paid more than $4 billion to people and families of vaccine injured people in the United States. Vaccines are liability-free products.
Since COVID vaccines were deployed, vaccine adverse event reporting systems around the world are recording a record number of injuries. By October 15th, 2021, adverse events reported worldwide passed 2,344,240 for COVID vaccines alone in the World Health Organization (WHO) reporting system VigiAccess.
Many researchers have also pointed out the potential for underreporting as well. According to multiple studies, it’s known that serious adverse reactions to prescription drugs, for example, are extremely underreported, perhaps up to 95 percent.
But what about serious adverse reactions to vaccines? A study published on October 7, 2021 in the Journal Toxicology Reports estimates that underreporting of deaths as a result of the COVID vaccines may have resulted in a number 1000 times less than what the actual number is.
They also cite a widely distributed Harvard Pilgrim study published in 2010 which suggested that less than 1 percent of vaccine injuries are reported. This includes serious adverse reactions.
Approximately 50 percent of COVID vaccine injuries reported to the Vaccine Adverse Events Reporting System within the last 30 year are all from COVID shots. As of October 15, 2021, Vaccine Adverse Events Reporting System (VAERS) recorded 122,833 serious adverse events, of those 17,128 resulted in death, post administration of COVID vaccines. This includes cases from around the globe, not just the US. You can look up the latest numbers here.